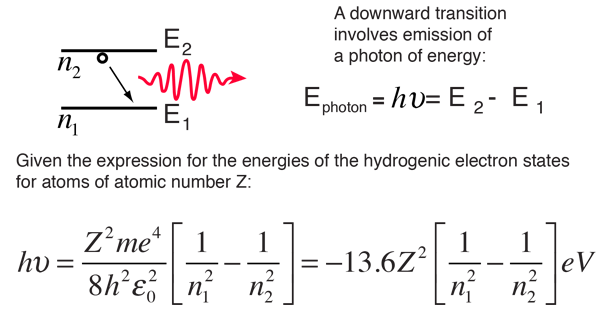
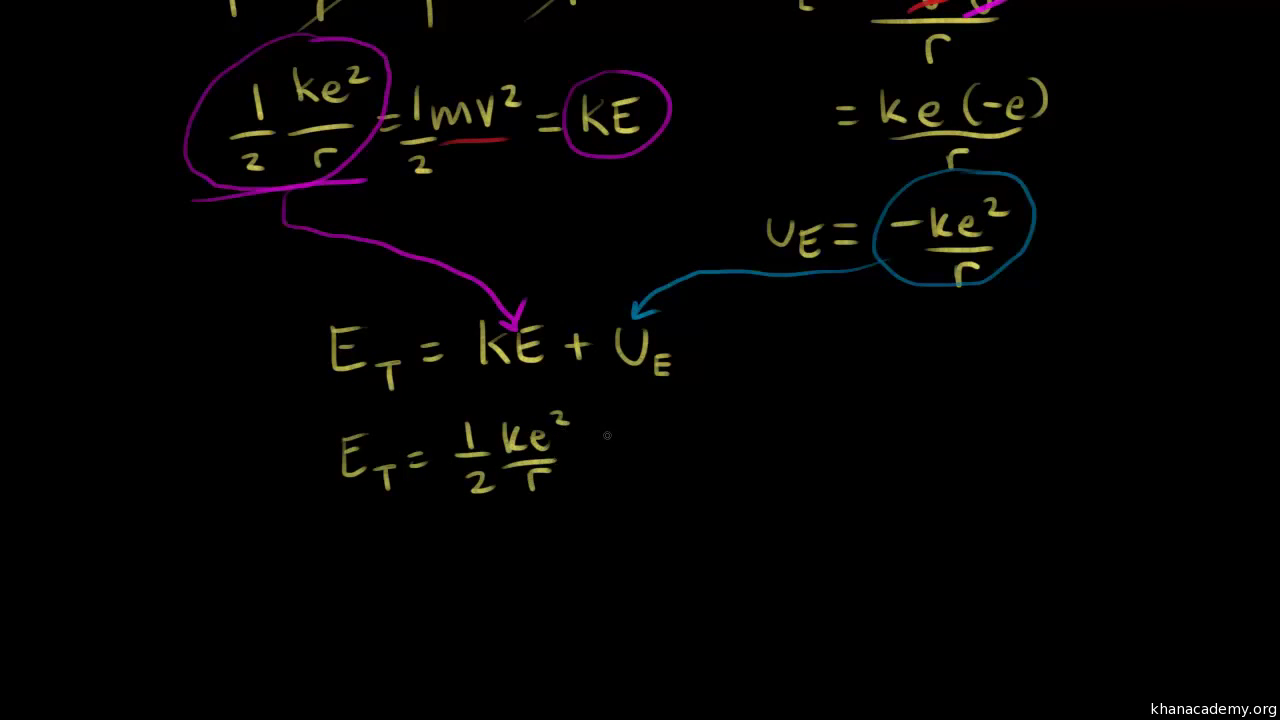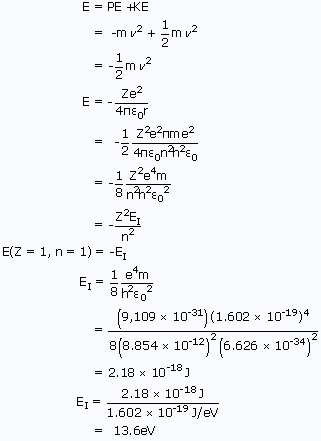Calculate the energy change of an electron in a neutral hydrogen atom moving from the n = 1 state to the n = 4 state. Answer in aJ. Why was it essential
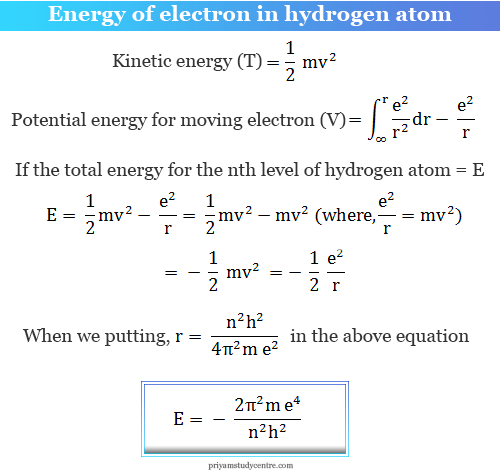
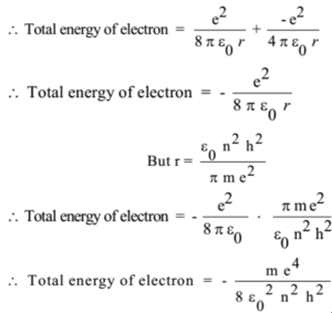
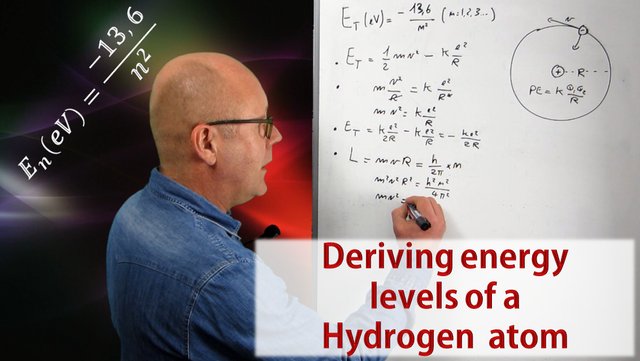
Derive an expression for the total energy of an electron in any orbit of a hydrogen atom in accordance with Bohrs atomic model

Using rutherford model of atom derive an expression for the total energy of the electron in hydrogen - Brainly.in
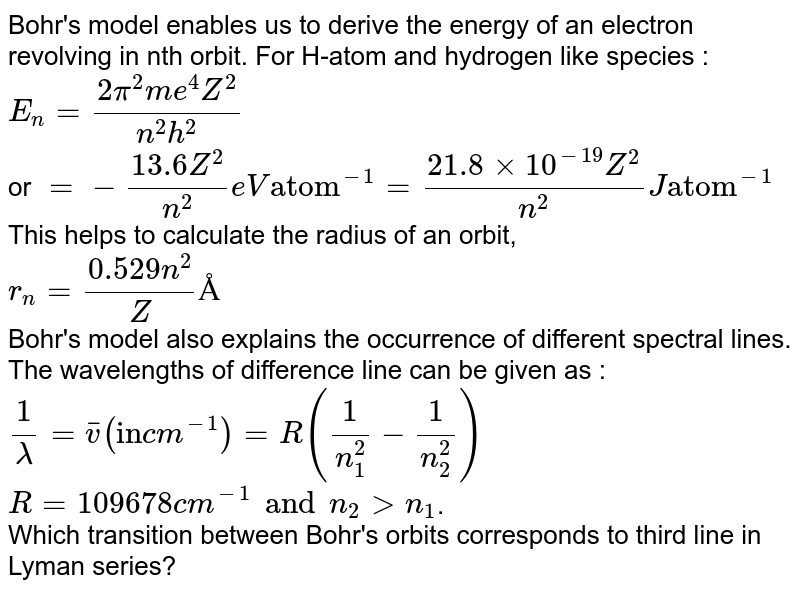
Bohr's model enables us to derive the energy of an electron revolving in nth orbit. For H-atom and hydrogen like species : En = (2 pi^2 m e^4 Z^2)/(n^2 h^2) or = - (